- Solutions
- AI Data and Analytics AI Data and Analytics
- Demo Center
- Resources
- Company

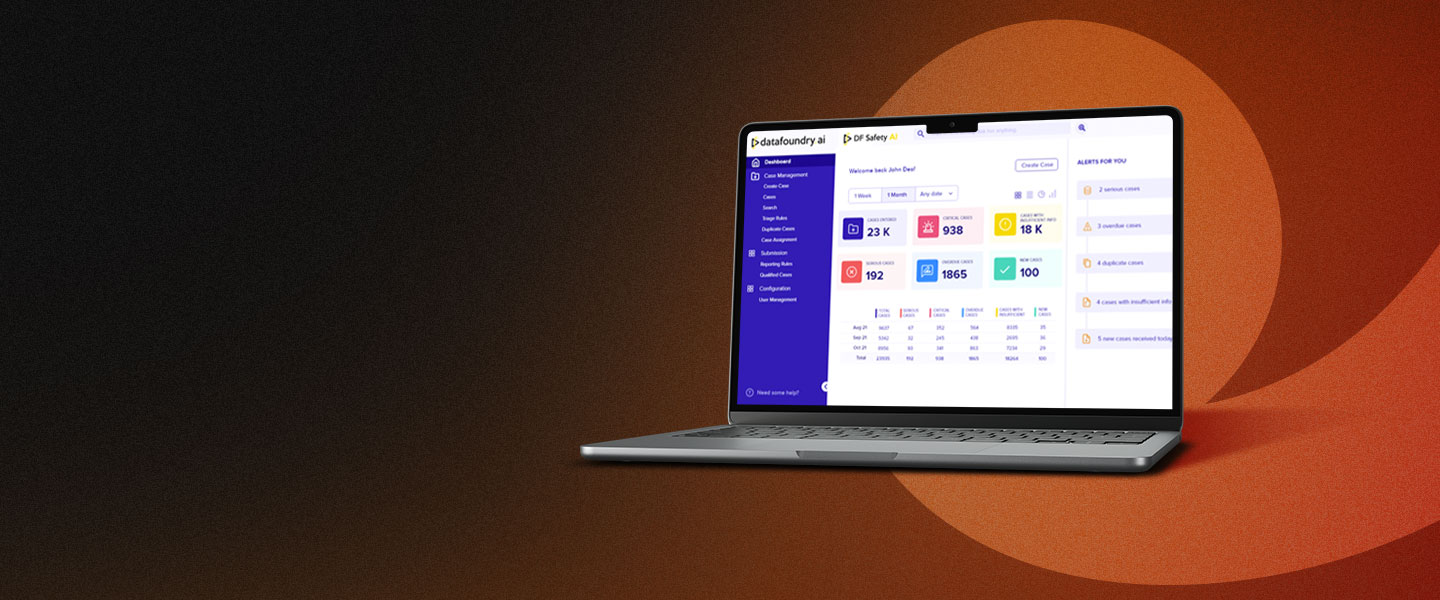

Transform the safety vigilance of your Life Sciences and Health Care products with our advanced AI Safety Platform. Our integrated modular Multi-Vigilance Platform caters to Pharma, Cosmetics, Devices, and Vaccines, offering streamlined safety solutions that drive efficiency, accuracy, and rapid insights.
60% Reduction in Implementation Time
40% Reduction in Total Cost
3X Efficiency with Process Automation
Anywhere-Any Cloud Deployment
DF Safety AI offers a comprehensive set of automation features in a single platform, enabling efficiencies and compliance in end-to-end safety vigilance operations. Key functionalities include automated case intake options from narratives, documents and E2B XML feed, triage and validity rule configuration, seriousness prediction, automated causality assessment, expectedness and listedness checks, modular literature monitoring, integrated medical term coding, signal and risk management, auto narrative and report generation, compliance alerts, E2B R2 & R3 export/import, automated submissions management and the ability to configure workflows and roles.
ExploreDF Signal AI brings AI innovation to Signal and Risk Management processes across pharma, vaccines, devices and cosmetics. It offers market leading features such as: signal detection from multiple agency databases, AI predictions for disproportionality analysis, active and passive surveillance, configurable clinical risk flags, alerts, notifications and stratifications. With an intuitive user experience, seamless data integration, and advanced analytics, DF Signal AI enables efficient collaboration and reporting. With AI-driven functionalities, DF Signal AI, combines comprehensive data analysis with advanced analytics. This powerful tool not only helps identify potential risks and signals in real-time, but also facilitates efficient benefit-risk management, regulatory compliance and informed decision-making.
ExploreDF Literature Monitor solves the key challenges in literature surveillance for safety. In just a few minutes users can set-up monitoring projects for drug-AE combinations. The intelligent search algorithm will then download articles from multiple databases to enable quick review and extraction of adverse events. With DF Literature Monitor, the entire process of article search, assessment and AE extraction is automated. Workflows for procurement enables purchase of paid articles. The machine translation capabilities allow monitoring of local literature databases. Translated articles and Embase articles can be uploaded into the tool as well, making this the most comprehensive literature monitoring solution in the market. DF Literature Monitor reduces manual effort, while ensuring seamless data transfer in suitable AE form/E2B feed to be submitted to a safety database of your choice.
ExploreKey challenges we solve:
Regulatory Risks triggered by scattered data in legacy systems.
Ineffective Signal Detection & Management
Inability to accurately predict future outcomes that can be trusted.
Across the life sciences industry, major players are grappling with billions of dollars in product recalls, labelling issues, and failed clinical trials attributed to safety concerns. The pressing need for transformation in safety vigilance practices has never been more evident. Digital transformation is critical to driving automation and elevating data quality in the key area of Safety Vigilance, a challenge that comes with many obstacles.
High cost of data transformation
Long cycle time to realise benefits
Organisational change management
As a premier technology company focused on using data and automation for ensuring safer products and better health outcomes, we cater to pharmaceutical, medical devices, cosmetics, nutraceuticals, CROs and research institutions. Our specialized solutions eradicate manual processes, slashing costs and timeframes. Embrace data-driven automation for decisive analysis, precise conclusions, and a competitive edge.
At Datafoundry, we're more than just a solution provider. We're your partner in achieving data-driven excellence. Our AI-powered insights and automation propel you towards faster, more informed decisions. Maximize your competitive advantage and embark on a journey of transformation with Datafoundry.

World’s Most Innovative AI Tools - 2023

Best use of Machine Learning 2023

Top 10 Data Science Startups in India for 2023

NASSCOM’s AI Gamechangers Award 2022

10 Most Recommended AI Startups - 2019
