- Solutions
- AI Data and Analytics AI Data and Analytics
- Demo Center
- Resources
- Company
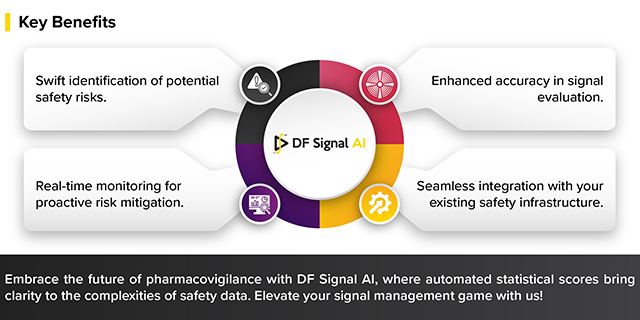
In the intricate world of pharmacovigilance, having swift access to comprehensive statistical scores is paramount.…
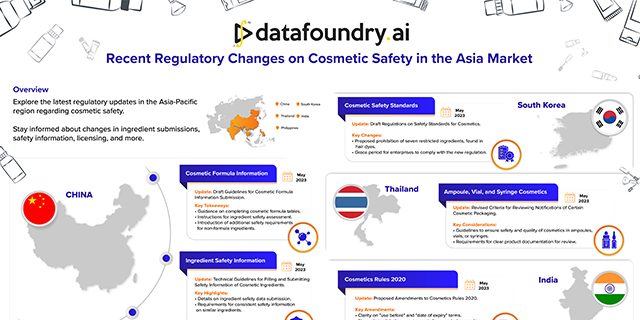
Explore the latest regulatory updates in the Asia-Pacific region regarding cosmetic safety. Stay informed about…

Gain valuable insights into the Modernization of Cosmetics Regulation Act (MoCRA) of 2022 through our…

Read our article "The Potential of Automation and AI/ML in Causality Assessment for Safety Vigilance,"…

Are you a technology head, regulatory leader, or cosmetic safety professional striving to enhance the…

Read about the challenges posed by data gaps in developing reliable machine learning models for…

The infographic showcases the game changing features of DF Safety AI Ver 4.0 that helps…
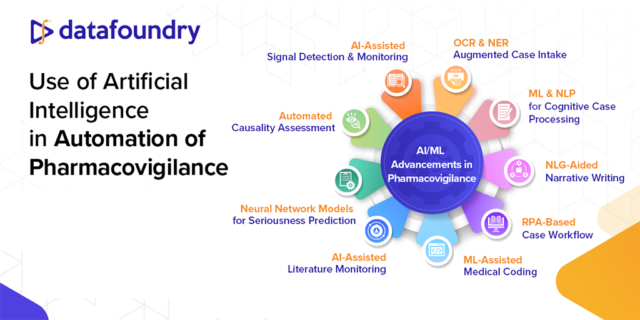
Read about how AI/ML has the potential to increase efficiency, accuracy and consistency in pharmacovigilance…
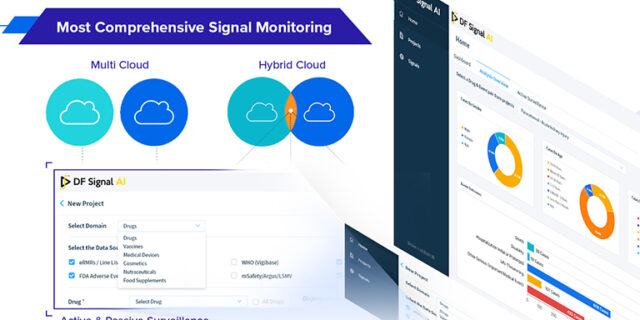
The infographic showcases the key features and benefits of DF Signal AI and how it…