
Introduction:
The drug development process for hit-to-lead molecules relies inevitably on the knowledge of adequate In drug development process, the hit-to-lead phase, also referred to as lead generation, is a critical stage where the first set of compounds that demonstrated promising activity against the target are characterized. It typically occurs following the completion of target validation, assay development, and high-throughput screening studies and relies inevitably on the knowledge of adequate safety and efficacy of the drug candidates prior to approval. This information can be obtained by mining biological databases containing structure – activity relationships as well as literature databases containing safety reports of drugs tested in pre-clinical & clinical trials. Literature monitoring process is vital to understand, analyse and develop scientific claims for drug design, development and validation.
With the exponential increase in number of articles published each day, it is challenging for the pharmaceutical companies to process this information without the power of automation. The traditional approach to detecting ADRs – literature review – is time-consuming and labor-intensive.
Literature monitoring can be automated using artificial intelligence (AI) and machine learning (ML), which can reduce the time it takes to detect new drug safety information.
Automating literature monitoring
Pharmacovigilance experts read through study reports, case studies and other literature to watch for any drug safety risks. This process is often time-consuming and can be prone to human error. To automate the process, Datafoundry has developed a machine learning platform that ingests data from a growing number of scientific sources and automatically flags potential drug safety risks. The platform has been trained on a large dataset of safety information and is able to detect risks that are often missed by human readers.
In pharmacovigilance, literature monitoring is crucial, and Datafoundry helps to automate it
Literature monitoring is an important activity in pharmacovigilance, it is the science and practice of identifying, assessing, and minimizing the risks of adverse effects of drugs.
Pharmacovigilance experts continuously monitor the scientific literature for new information on drugs—both those that are already on the market and those that are being developed. This information can help them identify potential drug safety issues early and take appropriate action to minimize any risk to patients. The sheer volume of scientific papers published every day can make this task impossible for humans to do manually. That’s where AI/ML comes in.
Datafoundry’s automated literature monitoring tool continuously scans the scientific literature for new case reports, articles on drugs, analyses them for drug safety related risks, and alerts pharmacovigilance experts of any potential issues. This enables them to focus their time and resources on the most important articles and take quick action if needed.
Monitoring literature through automation offers several benefits
There are many benefits of automating literature monitoring for drug safety vigilance. Some of the key benefits are:
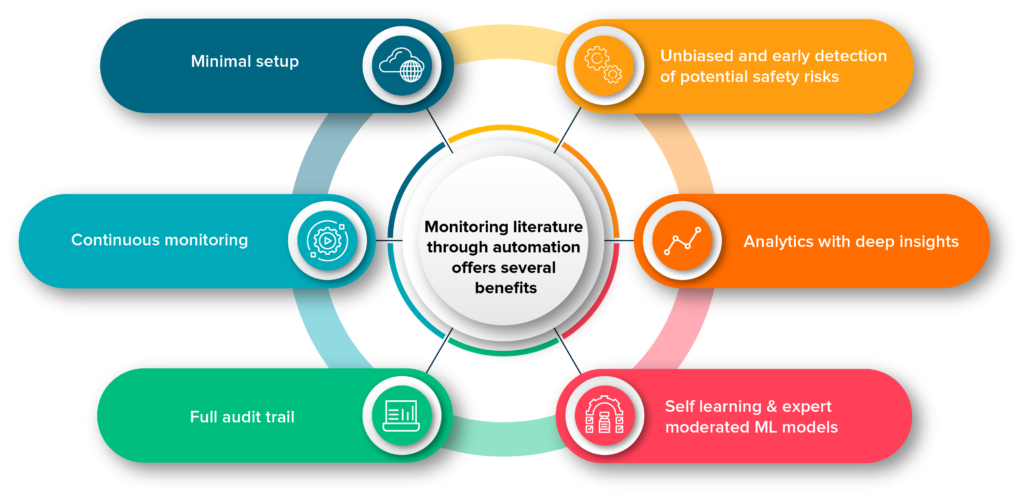
• Automated systems are unbiased and can identify potential safety risks that manual reviewer might miss
• Provide analytical charts from the gathered data automatically to gather deep insights pertaining to the query drug and parameters where its safety is dubious
• Machine learning models learn from the data modified by Quality control professionals that will enhance the accuracy of models and reduce the burden of manual efforts
• Provides full audit trail of text mining actions, ensuring pharmacovigilance compliant reporting to regulators.
• Automated systems never get tired and can work round the clock
• Cloud and web-based services requires minimal setup.
How does Datafoundry’s literature monitoring tool work?
DF Literature Monitor from Datafoundry is an AI-powered literature search and case extraction solution for safety vigilance. DF Literature Monitor is a cloud based SAAS product that integrates with any Safety database system. It scrapes information from multiple internal & external Databases, Manual uploads to provide a single bucket of Abstracts/FTA collection.
Datafoundry’s software conducts a periodic search of all published literature, for a list of given drugs to identify new safety information. The system then extracts the relevant case information from the articles and stores it in a safety database for further evaluation. This process is fully automated, so it can be completed quickly and efficiently. The software also integrates with existing safety databases, so data can be easily accessed and analysed.
Case study: How Datafoundry’s literature monitoring tool helped a pharmaceutical company
Let’s take a look at one to see how DF Literature Monitor helped a pharmaceutical company with their safety vigilance efforts.
The company was dealing with an issue where a tropical drug with retinoid was causing acute hair fall as a side effects. They needed to find any and all related literature as quickly as possible in order to assess the situation and take appropriate action. The team turned to Datafoundry’s AI-powered literature search and case extraction solution.
Datafoundry team helped them set up a configurable search strategy in the DF Literature Monitor, that would not only provide an on-the-spot monitoring of literature search, but also would periodically monitor the growing number of scientific sources. Cutting edge AI technology and self-learning ML models of DF Literature Monitor allowed full audit trail of text mining actions, ensuring pharmacovigilance compliant reporting to regulators.
The pharma company was able to locate all of the relevant articles related to the drug and its potential side effects while staying compliant.
Final thoughts:
Literature monitoring is an important process in drug safety vigilance. Automating the process can bring many benefits, including improved speed, accuracy and efficiency. Datafoundry’s literature monitoring tool is a powerful and user-friendly solution that can help pharmaceutical companies meet their literature monitoring needs.

