
The life sciences industry is constantly evolving and seeking new ways to improve the safety and efficacy of medicines. One crucial aspect of this is safety signal management, which involves identifying potential safety risks associated with specific drugs and taking the necessary measures to mitigate these risks.
Signal and Risk management activities are critical from a patient safety and regulatory perspective to help establish the benefit-risk profile of a drug or treatment regimen. The collection and analysis of thousands of individual safety case reports (hundreds of thousands if one considers vaccines) has been largely a manual effort, and sometimes, the effort impacts the submission timelines for aggregate safety reports. Signal detection tools have mostly moved the challenge from Excel spreadsheets to Signal tools that mimic a spreadsheet, though with some automation for applying statistical methods.
In this context, using Artificial Intelligence (AI) and Machine Learning (ML) methods promises to transform signal detection and risk management by optimizing the process and enabling a much faster and more automated signal detection approach, better quality control, and collaborative risk management. The table below provides a comparison between traditional and AI-powered signal management approaches.
Comparing traditional signal management methods and AI-powered methods
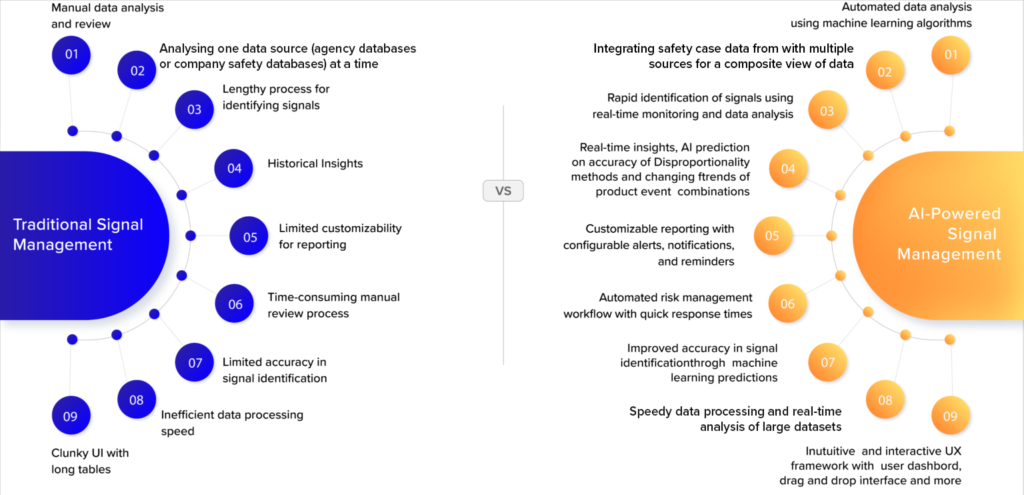
Combining the algorithms used for generating statistical scores on a large number of safety cases, the ease of use required for modern web applications, and a domain-centric approach for qualitative signal detection using line listings and existing knowledge, the team at Datafoundry has built DF mSignal AI – to leverage the power of AI/ML and transform signal and risk management of medicines, cosmetics, vaccines, and medical devices.
DF mSignal AI uses advanced analytics, including frequentist and Bayesian statistics, to identify potential risks and provide predictions based on a composite signal score. This score is generated by analyzing data from multiple databases, including regulatory agencies, clinical trials, and literature sources, and incorporating relevant clinical flags for specific product-event combinations.
One key trend in the future of signal management is the shift towards active surveillance, which involves actively monitoring and collecting data on potential safety risks. DF mSignal AI is at the forefront of this shift, providing “Data as a Service” for the most comprehensive active surveillance by using data from publicly available sources such as FAERS, EMA, Health Canada, etc., and updating it regularly. DF mSignal AI offers active (data-on-the-go) and passive surveillance for Drug Event Combination (DEC) monitoring. The technology also features a user-friendly drag-and-drop interface, allowing easy customization of views, alerts, and notifications. This, combined with the ability to integrate variables from multiple databases, makes DF mSignal AI a versatile and powerful tool for signal management.
As the life sciences industry continues to grow and evolve, artificial intelligence will become increasingly important.
According to a research report from The Business Research Company the global Artificial Intelligence (AI) in pharma market is expected to grow from $2,895.5 million in 2025 and reach $9,142.7 million in 2030.2
DF mSignal AI Workflow
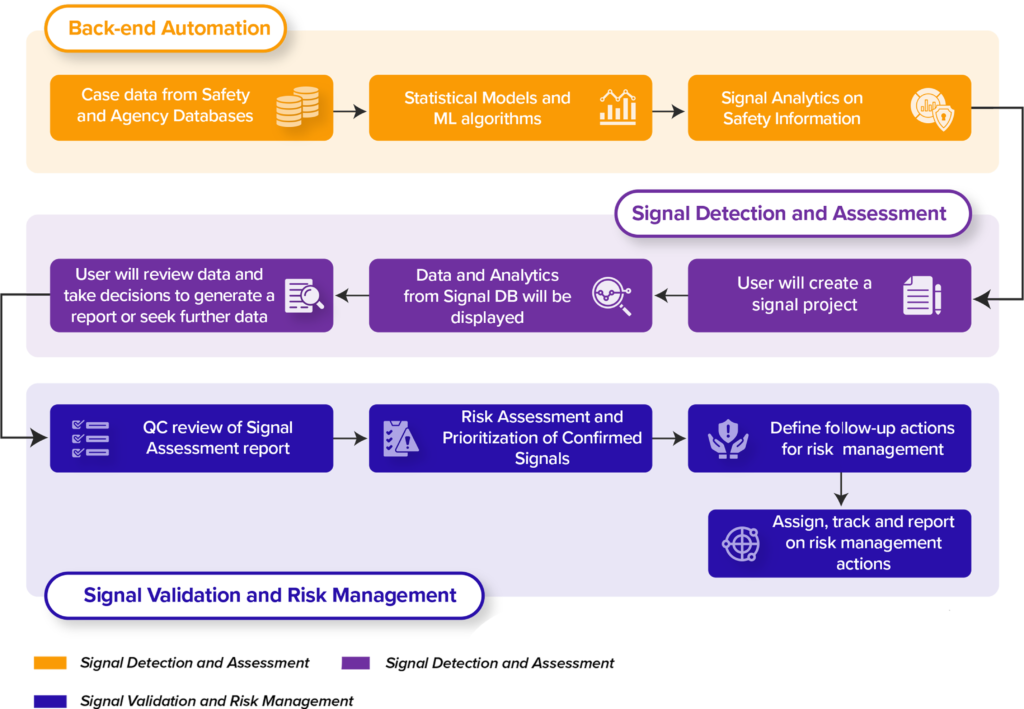
Another significant trend is the increasing use of big data analytics, which allows for analyzing large and complex datasets in real time to complement and optimize the practice of safety signal detection1. DF mSignal AI is well-equipped to meet these demands, with the ability to store statistical results and track risk and signal review through its Signal Management and Risk Management workflows.
The future of signal management is likely to see a greater emphasis on collaboration and information sharing across the industry. DF mSignal AI supports this trend by offering advanced report-writing capabilities, including integration with safety databases, literature monitoring, and regulatory intelligence monitoring tools.
In conclusion, with the growing importance of artificial intelligence in the pharmaceutical industry, DF mSignal AI is poised to play a significant role in shaping the future of signal management with capabilities for providing advanced analytics and real-time monitoring capabilities that allow pharmaceutical companies to quickly identify and mitigate potential safety risks. The benefits of automation tools in signal management are reflected in the increased speed of data analysis, improved signal identification accuracy, and increased reporting customizability. As AI technology advances, these tools will continue to revolutionize signal management, making it a safer and more effective process for patients, healthcare providers, and regulatory agencies.
Reference:
1.Heba Ibrahim, A. Abdo, Ahmed M. El Kerdawy, A. Sharaf Eldin,Signal Detection in Pharmacovigilance: A Review of Informatics-driven Approaches for the Discovery of Drug-Drug Interaction Signals in Different Data Sources,Artificial Intelligence in the Life Sciences,Volume 1,2021,100005,ISSN 2667-3185,https://doi.org/10.1016/j.ailsci.2021.100005.
2. AI In Pharma Market 2021 – Global Forecast To 2030, Published: September 2021
https://www.thebusinessresearchcompany.com/report/ai-in-pharma-market

