Introduction
Identifying adverse events from medical literature is one of the key tasks in pharmacovigilance (PV). It is also one of the most complex.
In pharmacovigilance, patient safety and regulatory compliance are paramount, and correctly identifying adverse events from the vast ocean of medical literature is akin to finding a needle in a haystack. Let’s delve into the intricacies of this process, understand the challenges it presents, and explore how DF Literature Monitor is revolutionizing the landscape.
The Overwhelming Volume of Information
The first challenge is the sheer volume of information. Medical literature is expanding at an exponential rate, inundating researchers and pharmacovigilance professionals with an overwhelming amount of data. 1. This means that pharmacovigilance professionals have to deal with an enormous amount of information on a daily basis, which poses significant challenges for finding relevant and reliable data on adverse events.
Moreover, the literature sources are heterogeneous and scattered across different databases, journals, and languages, requiring extensive search strategies and manual screening2. In fact, even the most meticulously constructed searches often yield only a minuscule fraction of relevant references. Imagine sifting through mountains of data to find that needle—the relevant information that could impact patient safety and drug regulatory decisions.
The Menace of Duplicate Articles
Another major challenge in adverse event identification from medical literature is the presence of duplicate articles, which can lead to over-reporting and inconsistency of data. Duplicate articles are those that report the same or overlapping information on the same study or case report but appear in different sources or formats. It’s a menace that can’t be ignored, as on average, they account for around one-third of the references retrieved during the monitoring process. This not only adds to the workload but also poses a challenge in distinguishing genuine findings from redundant data.
Regulatory Rigor and Quality Management
Adverse event identification from medical literature is not only a complex task, but also a highly regulated one. Pharmacovigilance professionals must comply with strict guidelines and standards from various regulatory authorities, such as the European Medicines Agency (EMA), the Food and Drug Administration (FDA), and the World Health Organization (WHO). These guidelines specify the requirements and procedures for conducting literature searches, screening articles, extracting data, reporting adverse events, and documenting quality measures. Failure to adhere to these regulations can result in serious consequences, such as fines, sanctions, or loss of market authorization3.
A Game-Changer for Adverse Event Identification
To overcome these challenges and streamline the process of adverse event identification from medical literature, Datafoundry has developed a cutting-edge solution: DF Literature Monitor.
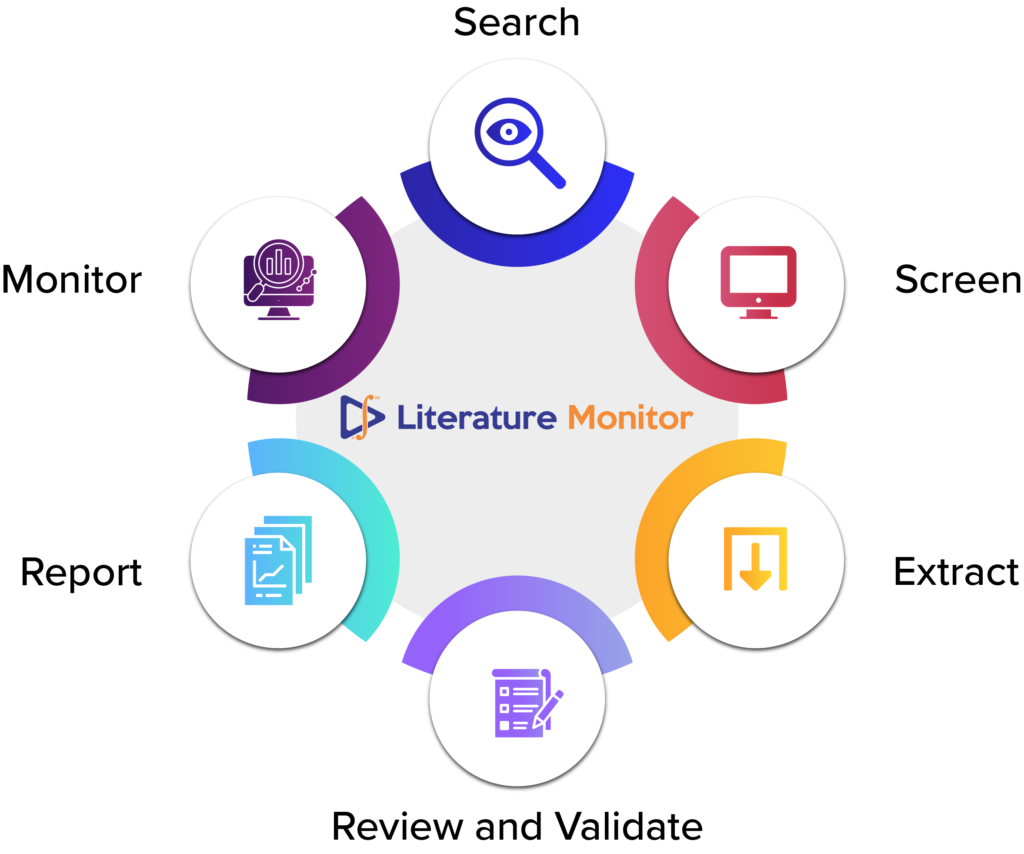
DF Literature Monitor is an automated literature monitoring tool that continuously scans the scientific literature for new case reports, articles on drugs, analyses them for drug safety related risks, and alerts pharmacovigilance experts of any potential issues. DF Literature Monitor leverages advanced natural language processing (NLP) and deep learning techniques to perform the following tasks:
- 1. Search: DF Literature Monitor uses sophisticated search algorithms to query multiple databases and literature sources for relevant articles on drugs and adverse events. It also applies filters and criteria to refine the search results and eliminate irrelevant or duplicate articles.
- 2. Screen: DF Literature Monitor employs state-of-the-art NLP models to analyze the content and metadata of each article, such as title, abstract, keywords, authors, affiliations, etc. It then classifies the articles into different categories based on their relevance and reliability for pharmacovigilance purposes.
- 3. Extract: DF Literature Monitor extracts key information from each article using advanced NLP techniques, such as named entity recognition (NER), relation extraction (RE). It identifies and extracts entities such as drugs, adverse events, patient characteristics, outcomes, etc., and auto populates the Minimum safety information (MSI) table.
- 4. Review and Validate: DF Literature Monitor provides a seamless user experience enabling user to view the article and the safety-related information on the same screen, thereby reducing error and increasing efficiency. The PV expert can view, edit and validate the safety information conveniently.
- 5. Report: Post validation by the expert, DF Literature Monitor generates structured and standardized reports for each article containing the extracted information. The case reports can be exported as a CIOMS or as E2B compatible R3 format, which can be further integrated with any safety database.
- 6. Monitor: DF Literature Monitor monitors the several literature databases continuously and updates its database with new articles and reports to ensure the user always has the latest information at their fingertips. It also provides dashboards and analytics tools for pharmacovigilance experts to monitor trends and patterns of adverse events across drugs, populations, regions, etc.
By automating these tasks, DF Literature Monitor reduces manual efforts, improves accuracy, and enhances decision making in pharmacovigilance. It also ensures compliance with regulatory requirements and quality standards by providing traceability and auditability of the literature monitoring process.
DF Literature Monitor is a game-changer for adverse event identification from medical literature. It is a powerful tool that enables pharmacovigilance professionals to keep up with the ever-growing volume of information, avoid duplication and inconsistency of data, and comply with regulatory rigor and quality management. With DF Literature Monitor, pharmacovigilance professionals can focus on what matters most: ensuring patient safety and drug efficacy.
References:
- 1. Golder S et al. Reporting of Adverse Events in Published and Unpublished Studies of Health Care Interventions: A Systematic Review. PLoS Med 2016; 13(9): e1002127.
- 2. European Medicines Agency. Medical literature monitoring. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medical-literature-monitoring
- 3. Golder S et al. The problems caused by duplicate publications and how to avoid them. Int J Clin Pract 2019; 73(11):e13400