
Safety concerns associated with therapeutic intervention(s) and immunizations can be detected, analyzed, and minimized only through early identification and having proper risk minimization measures throughout the life cycle of a drug.
Researchers face multiple challenges while designing the right strategies for early detection of safety signals. Safety concerns can arise throughout the lifecycle of a drug, broadly divided into pre-market clinical development and post marketing surveillance activities.
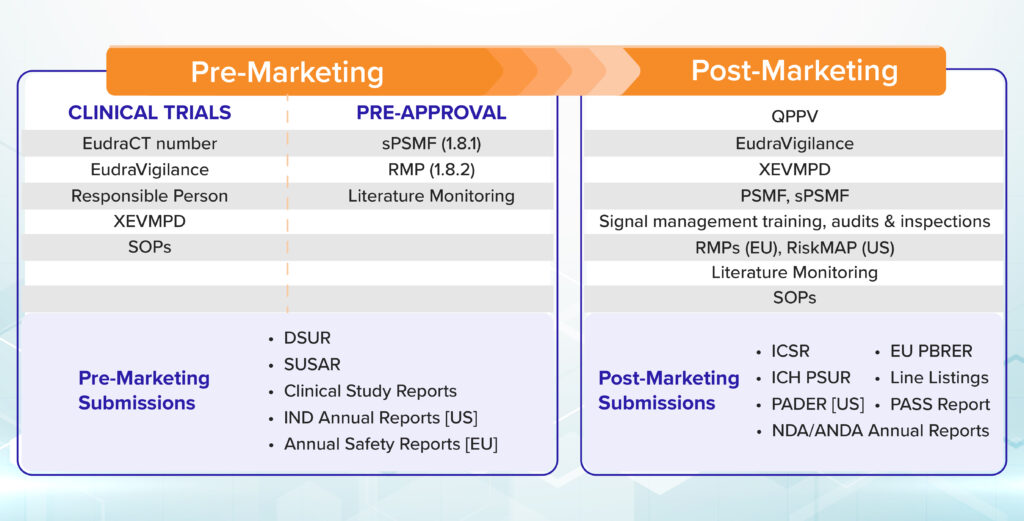
The pre-market safety assessments cannot reveal all safety concerns due to the limited and controlled nature of the studies. Hence, post marketing safety monitoring is critical to obtain real world safety issues.
The focus of pre-market safety assessment is to protect the research subjects enrolled in the trials, to decrease unnecessary spends the pharma companies may incur on novel therapeutic interventions, to explore new indications, ethnicity/population selection and doses/regimens selection. These early identifications will help the researchers/pharma companies make the medicines available faster to the patients while keeping the benefit-risk profile on the positive side by addressing the clinically significant safety information.
For the pre-market safety activities, a signal and risk management solution such as DF Signal AI can be coupled with a review of the published literature through DF Literature Monitor. The meta-analyses of clinical trials or drug class effect data, and analysis of the events associated with other products in the same therapeutic class can help identify premarket safety concerns effectively.
During the post-marketing phase of the drug life cycle, signal and risk management solutions serve as immensely helpful tools, if coupled with high-quality historical data on safety issues collected through standardized processes at various regulatory bodies across the world.
However, conventional signal and risk management solutions are unable to address the emerging challenges effectively. This is where an innovative, AI-assisted approach to Signal and Risk Management offered by DF Signal AI could help the researchers and analysts.
-
- 1. Increased number of AEs (Adverse Event) create a problem
sin assessing true safety issues. Longer intervals to find novel issues is another issue as the harm may have increased significantly by then DF Signal AI fetches deduplicated, standardized data from curated AE (Adverse Event) repositories, to detect clinically significant issues with the help of AI predictions.
- 1. Increased number of AEs (Adverse Event) create a problem
-
- 2. It is important to tag or flag clinically relevant risk flags to identify counterfeit, misuse and special interest cases for effective signal detection. The curated AE repository of DF Signal AI addresses these issues with the help of an ML framework for classification enablings users to create their own risk flags and derive signals from the same.
- 3. Organizational issues due to individual process variabilities could have an impact on causality assessment, completeness, and validity of reports. DF Signal AI addresses these issues at project creation level by enabling prioritized risk type which can be further confirmed on the basis of the signal assessment criteria such as strength of association, possible health impact, novelty of drug event combination and preventability.
In addition to mitigating the above inefficiencies and challenges, DF Signal AI utilizes data driven algorithms like Logistic Regression and Random Forest to let the user make more informed decisions on applying statistical algorithms to identify a true signal.
The application also predicts which algorithms (at selected prioritization threshold based on used quantitative datasets) are giving better signal accuracy in detecting statistically significant events at 95% confidence intervals, with lesser false positives. This allows the DF Signal AI users to make informed observations around signal detection with additional stratifications as required for qualitative and quantitative analysis of data and risk assessment in a continuous and systematic manner.
Advantages of DF Signal AI
DF Signal AI has been conceptualised and designed by industry experts to serve as a comprehensive solution that leverages data sciences and places the needs and user experience of the safety signal analyst at the forefront. DF Signal AI offers the below advantages compared to conventional signal detection tools in the market:
-
- • Users can define the signal and risk management strategy on available data sources, decide on statistical algorithms to find a true signal through ML predictions and create a signal schedule/calendar.
-
- • The application uses near real time data for quantitative and qualitative analysis which is automated to update periodically and integrated to visualize the identifying trends and areas of interest.
-
- • Users can plot different tree maps and perform cluster analysis based on data related to MedDRA terms and ATC (Anatomical Therapeutic Chemical) classifications. This helps detect drug class effects, and identifying the relationship for a greater or lesser likelihood of drug and event combinations based on demographics or other stratifications as applied.
-
- • DF Signal AI, provides users with the ability to manage the risk related findings (signal findings) after quality approval in the workflow. Risk assessment can be performed based on FMEA (Failure Mode Effect Analysis) and an RPN (Risk Priority Number).
-
- • Signal confirmation can be done based on the signal teams’ decisions, sending signal details to relevant stakeholders and capturing the decisions and actions through meeting minutes within the application. Users can track the risk management actions to closure, thus avoiding the use of separate systems, unlike other solutions in the market.
In conclusion, the DF Signal AI solution can help address the various challenges comprehensively to manage the signal and risk management plans of many types of medicinal products like drugs, cosmetics, nutraceuticals, vaccines (biologics) and devices simultaneously and ensure compliance with local and country specific regulatory requirements for a well-controlled product life cycle and product safety.
To request a discussion with the Product team and a product demonstration, please click here.
