In today’s high-stakes pharmaceutical environment, patient safety is no longer merely a regulatory checkbox—it is the cornerstone of public trust, clinical responsibility, and operational excellence. As medicinal products enter global markets faster than ever, the challenge of effectively monitoring adverse drug reactions (ADRs) has intensified. Signal detection has evolved from manual review to algorithm-driven surveillance, demanding a strategic and systematic response. The European Medicines Agency (EMA) has addressed this shift by updating its Good Pharmacovigilance Practice (GVP) Module IX, establishing a new gold standard in signal management.
Understanding the Shift: What Changed with GVP Module IX Revision 1?
Originally introduced in 2012, GVP Module IX provided a foundational framework for detecting and managing safety signals. However, with increased data complexity and heightened global expectations, a comprehensive revision was rolled out in November 2017, reinforcing the need for continuous, real-time pharmacovigilance.
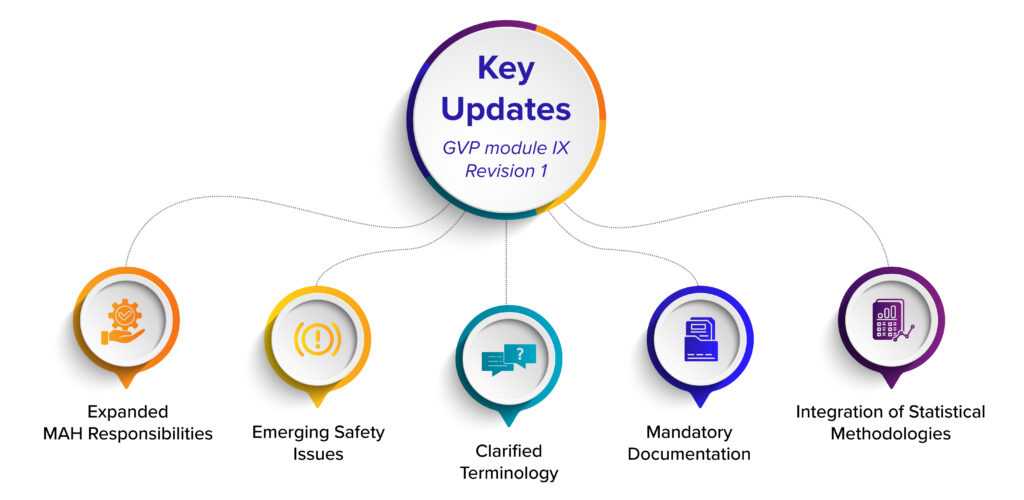
Key updates include:
1. Expanded MAH responsibilities: Marketing Authorisation Holders (MAHs) must now monitor not only internal safety databases but also EudraVigilance for early identification of validated signals.
2. Emerging safety issues: New or worsening risks must be reported to regulatory authorities within three working days of the MAH becoming aware of information that constitutes an emerging safety issue.
3. Clarified terminology: Standard definitions—such as “validated signal” and “signal prioritisation”—create a shared vocabulary across industry stakeholders.
4. Mandatory documentation: Documented procedures, decision rationales, and audit trails are now compulsory, ensuring transparency and regulatory readiness.
5. Integration of statistical methodologies: Advanced techniques—such as Proportional Reporting Ratio (PRR) and Bayesian analysis—are formally recognized and detailed in Addendum I.
The Mounting Complexity of Signal Management
Modern pharmacovigilance is overwhelmed by the volume and variety of safety data. From clinical trials and electronic health records (EHRs) to patient forums and wearable technologies, each source holds the potential to reveal critical safety information—provided it can be accurately analysed and contextualized.
Four primary drivers of complexity:
-
-
-
- ➣ Data explosion. Real-world evidence (RWE), unstructured patient narratives, and global reporting systems require advanced tools for harmonization and synthesis.
- ➣ Evolving surveillance models. Passive collection is no longer sufficient; regulators demand active monitoring of real-world datasets, epidemiological studies, and registries.
- ➣ Stricter regulatory oversight. Inspection findings often cite incomplete documentation and inconsistent workflows, highlighting the need for systematic, auditable processes.
- ➣ Methodological rigor. The push to adopt complex statistical models necessitates analytical capabilities beyond traditional pharmacovigilance
- ➣ Data explosion. Real-world evidence (RWE), unstructured patient narratives, and global reporting systems require advanced tools for harmonization and synthesis.
-
-
Why Artificial Intelligence and Machine Learning Are Game-Changers
Artificial Intelligence (AI) and Machine Learning (ML) are reshaping the pharmacovigilance landscape. Tools such as PRR, Information Component (IC), and Empirical Bayes Geometric Mean (EBGM) are now enhanced by AI models to improve signal detection with greater accuracy and sensitivity.
AI contributions across the signal detection lifecycle:
-
-
- ➣ Machine Learning (ML). Excels at recognizing patterns in large, diverse datasets—including clinical data, spontaneous reports, and EHRs—identifying statistically significant correlations that might otherwise go unnoticed.
- ➣ Machine Learning (ML). Excels at recognizing patterns in large, diverse datasets—including clinical data, spontaneous reports, and EHRs—identifying statistically significant correlations that might otherwise go unnoticed.
-
-
-
- ➣ Natural Language Processing (NLP). Extracts structured insights from unstructured text such as physician notes, literature, and call transcripts, enabling the identification of drug-event relationships.
- ➣ Natural Language Processing (NLP). Extracts structured insights from unstructured text such as physician notes, literature, and call transcripts, enabling the identification of drug-event relationships.
-
-
-
- ➣ Real-time monitoring. Transforms signal detection from a reactive to a proactive process, enabling near-instant alerts and faster regulatory actions.
- ➣ Real-time monitoring. Transforms signal detection from a reactive to a proactive process, enabling near-instant alerts and faster regulatory actions.
-
-
-
- ➣ Predictive analytics. Anticipates safety risks by analysing historical trends, patient demographics, co-medications, and even genetic data.
-
A Reality Check: Why AI Is Not a Silver Bullet—Yet
While the promise of AI in pharmacovigilance is immense, its widespread operational use remains limited. Eight years after the implementation of GVP Module IX Revision 1, several persistent challenges impede full-scale adoption.
Technical promise v/s practical hurdles:
-
-
-
- ➣ Lack of data standardization: Pharmacovigilance data is scattered across incompatible formats. Without harmonized standards, training AI models becomes difficult. Integration across legacy systems remains a major barrier, making real-time signal detection nearly impossible without seamless data flow.
- ➣ Lack of data standardization: Pharmacovigilance data is scattered across incompatible formats. Without harmonized standards, training AI models becomes difficult. Integration across legacy systems remains a major barrier, making real-time signal detection nearly impossible without seamless data flow.
-
-
-
-
- ➣ Algorithm bias and interpretability: AI and ML models depend fundamentally on the quality and integrity of their training data. Incomplete or biased datasets can generate false positives—or worse, miss critical signals. Moreover, many ML models function as “black boxes,” producing risk scores without clear explanations. This lack of transparency undermines confidence for both regulatory bodies and internal QA teams.
- ➣ Regulatory and validation challenges: Although GVP Module IX Addendum I encourages the adoption of statistical models like PRR and Bayesian approaches, a global regulatory consensus on AI model validation remains elusive. Without harmonized guidelines for algorithm performance, auditability, and lifecycle monitoring, companies are hesitant to scale AI in highly regulated environments.
- ➣Data privacy and ethical complexities: Advanced AI models often require access to highly sensitive patient data. Ensuring compliance with frameworks such as the General Data Protection Regulation (GDPR), managing patient consent, and building secure cloud systems adds another layer of complexity—especially for organizations operating across multiple jurisdictions.
- ➣ Algorithm bias and interpretability: AI and ML models depend fundamentally on the quality and integrity of their training data. Incomplete or biased datasets can generate false positives—or worse, miss critical signals. Moreover, many ML models function as “black boxes,” producing risk scores without clear explanations. This lack of transparency undermines confidence for both regulatory bodies and internal QA teams.
-
DF mSignal AI: Bridging the Gap Between Regulation and Innovation
To address these challenges, DataFoundry has developed DF mSignal AI, an AI-powered signal detection and risk management platform purpose-built for the pharmaceutical industry. Grounded in real-world pharmacovigilance workflows and regulatory frameworks, it supports end-to-end compliance while streamlining complex operations.
Key capabilities:
-
-
- ➣ Automated disproportionality analysis. Utilizes AI-enhanced statistical methods — PRR, IC, and EBGM — to efficiently detect and validate safety signals, facilitating prompt identification of potential drug-related risks.
- ➣ Automated disproportionality analysis. Utilizes AI-enhanced statistical methods — PRR, IC, and EBGM — to efficiently detect and validate safety signals, facilitating prompt identification of potential drug-related risks.
-
-
-
- ➣ Risk stratification. Offers customizable risk flags and prioritization algorithms tailored to specific product portfolios and therapeutic areas, enabling targeted risk assessment and efficient resource allocation.
- ➣ Risk stratification. Offers customizable risk flags and prioritization algorithms tailored to specific product portfolios and therapeutic areas, enabling targeted risk assessment and efficient resource allocation.
-
-
-
- ➣ User-centric collaboration. Features intuitive dashboards that promote seamless communication among pharmacovigilance, medical, and regulatory teams, enhancing coordinated responses to safety concerns.
- ➣ User-centric collaboration. Features intuitive dashboards that promote seamless communication among pharmacovigilance, medical, and regulatory teams, enhancing coordinated responses to safety concerns.
-
-
-
- ➣ Integrated surveillance. Connects seamlessly to global regulatory databases — FAERS, EMA, and Health Canada — as well as internal systems, enabling real-time monitoring of adverse events for comprehensive pharmacovigilance coverage.
- ➣ Integrated surveillance. Connects seamlessly to global regulatory databases — FAERS, EMA, and Health Canada — as well as internal systems, enabling real-time monitoring of adverse events for comprehensive pharmacovigilance coverage.
-
-
-
- ➣ Audit readiness. Ensures full transparency and preparedness for regulatory inspections by meticulously documenting and time-stamping every action within the system.
- ➣ Audit readiness. Ensures full transparency and preparedness for regulatory inspections by meticulously documenting and time-stamping every action within the system.
-
-
-
- ➣LLM-driven reporting. Leverages large language models to automate the generation of comprehensive, regulatory-compliant safety reports, enhancing accuracy and efficiency in documentation.
- ➣LLM-driven reporting. Leverages large language models to automate the generation of comprehensive, regulatory-compliant safety reports, enhancing accuracy and efficiency in documentation.
-
-
-
- ➣Advanced infographics and visualization. Transforms complex pharmacovigilance data into interactive visual representations, facilitating clearer insights and more informed decision-making across safety and regulatory teams.
- ➣Advanced infographics and visualization. Transforms complex pharmacovigilance data into interactive visual representations, facilitating clearer insights and more informed decision-making across safety and regulatory teams.
-

By embedding explainability, fairness, and traceability into every AI-driven process—so that each signal detection, risk flag, and report generation step is accompanied by a clear, human-readable rationale—DF mSignal AI ensures transparent and trustworthy recommendations. Combined with accelerated signal recognition, seamless automation of repetitive tasks, integrated real-time surveillance, and audit-ready documentation, the platform provides comprehensive oversight at every stage of pharmacovigilance. Its flexible, cloud-native architecture scales effortlessly as safety data grows and adapts to evolving regulatory demands. In transforming routine compliance into a strategic advantage, DF mSignal AI empowers pharmacovigilance teams to proactively mitigate risks, achieve operational excellence, and ultimately improve patient outcomes.
Signal Management for the Next Decade
GVP Module IX is more than a regulatory framework—it is a call to action for the industry to prioritize patient safety through robust, data-driven systems. In this evolving landscape, companies must harness the power of AI to stay ahead of regulatory expectations and market demands.
DF mSignal AI is not just another tool—it is a strategic enabler built at the intersection of machine learning, regulatory science, and domain expertise. With its integrated, compliant, and intelligent approach, pharmaceutical companies can ensure that their signal management strategies are future-ready, driving both operational excellence and public trust.
Further Reading
- ➣ EMA Guideline on GVP Module IX (Rev 1)
European Medicines Agency. “Guideline on Good Pharmacovigilance Practices (GVP) Module IX – Signal Management (Rev 1).” November 2017.
🔗 Read the guideline (PDF)
- ➣ GVP Module IX Addendum I
European Medicines Agency. “Addendum I to GVP Module IX – Methodological Aspects of Signal Detection from Spontaneous Reports.” October 2017.
🔗 Download Addendum I (PDF)
- ➣ AI & Signal Detection in Pharmacovigilance
van Stekelenborg J., De Bruin M. L., Chapman S. C. E., et al. “Artificial Intelligence and Signal Detection in Pharmacovigilance: Current Uses, Limitations, and Future Directions.” Drugs – Real World Outcomes. 2025.
🔗 View on SpringerLink
- ➣ Opportunities & Challenges of AI in PV
Weber J. C., Nguyen M., Dufour J. F., Salvo F. “Application of Artificial Intelligence in Pharmacovigilance: Opportunities and Challenges.” Therapeutic Advances in Drug Safety. 2024;15.
🔗 Read on PubMed
- ➣ ADR reports Database
European Medicines Agency. “European Database of Suspected Adverse Drug Reaction Reports (ADRreports).” Accessed 2025.
🔗 Explore ADRreports
