In the world of safety vigilance, the meticulous task of literature surveillance is crucial for ensuring patient safety and health outcomes. However, the manual search and review of literature articles is one of the most time-consuming activities, especially considering the vast corpus of medical literature published across thousands of journals. The sheer volume of this medical literature, the presence of duplicate articles appearing in manual searches, and the need for precise data extraction and reporting create a cumbersome workflow that is exhaustive and susceptible to manual errors.
This is where AI-powered literature surveillance solutions like DF Literature Monitor come into play, redefining the solution by automating and streamlining the entire medical literature surveillance process. Here are ten ways an AI-powered literature surveillance solution can save you time and effort:
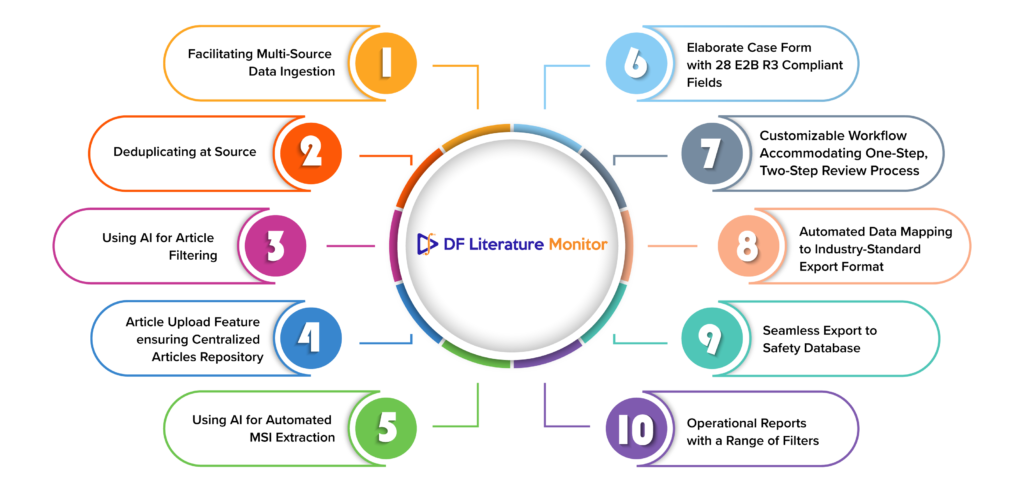
1. Facilitating Multi-Source Data Ingestion
An AI-powered literature surveillance solution can ingest articles from multiple databases and sources, such as PubMed and Embase. This multi-source data ingestion capability ensures comprehensive coverage of relevant literature without manual searches across various platforms. By automating this process, pharmacovigilance professionals can focus more on analysis than data collection. DF Literature Monitor offers an out-of-the-box integration of 25+ literature sources, including databases and journals, for scouring global and local medical publications.
2. Deduplicating at Source
Duplicate articles pose a significant challenge, often leading to over-reporting and data in consistency. An AI-powered tool can automatically identify and remove duplicate articles during ingestion. The system ensures that only unique articles are indexed by extracting and comparing metadata such as titles, abstracts, and authors. This reduces the workload and enhances data accuracy.
3. Using AI for Article Filtering
AI-driven solutions employ advanced algorithms to filter out irrelevant articles. Using natural language processing (NLP) techniques, the application can analyze and categorize articles based on relevance. This intelligent filtering process significantly reduces the number of articles that need to be manually reviewed, allowing Safety experts to concentrate on high-priority activities.
4. Article Upload Feature ensuring Centralized Articles Repository
DF Literature Monitor provides an article upload feature that allows users to keep all relevant articles for a specific product in one centralized location. This functionality ensures easy access and management of articles and helps maintain an organized one-stop repository of literature, streamlining the review and monitoring processes.
5. Using AI for Automated MSI Extraction
The extraction of Minimum Safety Information (MSI) is a critical aspect of safety vigilance. AI-powered tools utilize NLP techniques, including Named Entity Recognition (NER) and Relationship Extraction (RE), to automatically extract MSI from articles. This includes identifying key entities such as drugs, adverse events, and patient characteristics. Automated MSI extraction ensures accuracy and saves considerable time compared to manual extraction.
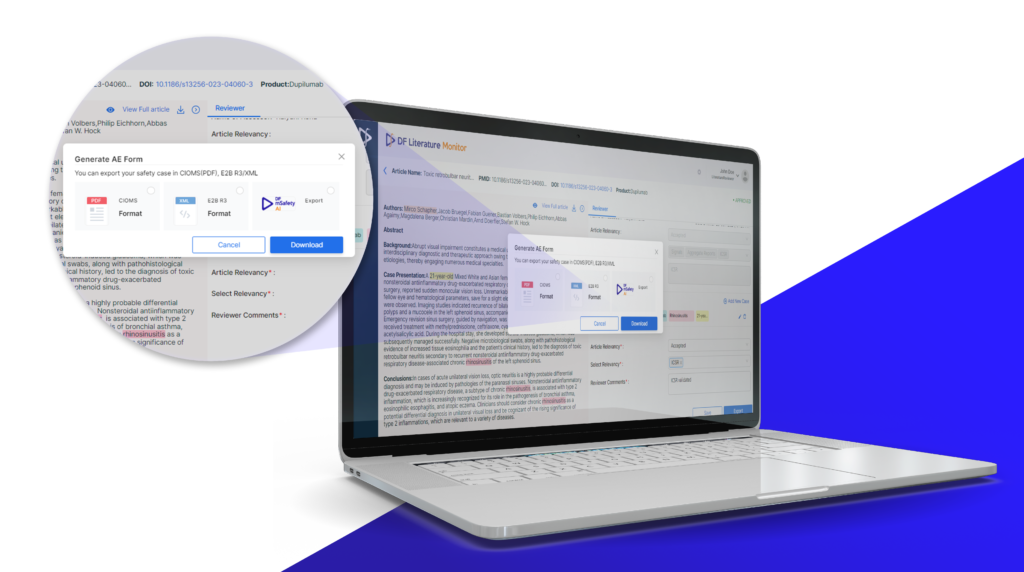
6. Elaborate Case Form with 28 E2B R3 Compliant Fields
DF Literature Monitor provides a safety case form with fields that are compliant with the E2B R3 standard. It contains 28 fields, ensuring all necessary information from a literature article is captured accurately and in the required format. This standardization facilitates seamless exporting and reporting to regulatory authorities and other stakeholders.
7. Customizable Workflow Accommodating One-Step, Two-Step Review Processes
A SaaS product must have customizable workflows to address the evolving needs for efficient literature surveillance. DF Literature Monitor offers customizable workflows that accommodate one-step and two-step review processes. This adaptability allows organizations to tailor the review process to their needs, enhancing efficiency and reducing the time required for literature review and approval while ensuring a thorough review process.
8. Automated Data Mapping to Industry-Standard Export Format
Mapping extracted data to industry-standard formats, such as CIOMS forms or ICH E2B R2/R3 compliant XML formats, is essential for regulatory compliance. DF Literature Monitor automates this data mapping process, ensuring accuracy and consistency. It eliminates the need for manual data entry, reduces errors, and saves significant time.
9. Seamless Export to Safety Database
Once the data is extracted and mapped, it must be integrated with safety databases. Therefore, seamless export capabilities, allowing case reports to be directly uploaded to safety databases, become crucial to any automated Literature monitoring system. This integration ensures that the latest safety information is available to safety teams in real-time, facilitating timely decision-making.
10. Operational Reports with a Range of Filters
Effective monitoring and reporting are vital for safety vigilance. Operational reports with filters, such as ICSR validity, article statuses, and reviewer information, allow users to generate customized reports based on various criteria. These reports provide valuable insights into trends and patterns in adverse events, helping safety experts document every step of the review process efficiently.
Conclusion
The manual processing of literature surveillance is burdened with challenges, including the vast volume of medical literature, duplicate articles, the need for precise data extraction and reporting, and the evolving regulatory landscape. AI-powered literature surveillance solutions, such as DF Literature Monitor, address these challenges by automating and streamlining the process.
These solutions save significant time and effort for pharmacovigilance professionals by facilitating multi-source data ingestion, deduplicating articles at the source, using AI for article filtering and MSI extraction, and providing features such as article uploads, customizable workflows, automated data mapping, seamless export to safety databases, and comprehensive operational reports.
Embracing AI-powered literature surveillance enhances efficiency, ensures compliance with regulatory requirements, and improves the overall quality of safety vigilance activities. PV experts can ensure better patient safety and health outcomes by redirecting focus from arduous tasks to higher-value activities.
Datafoundry’s DF Literature Monitor is a testament to the immense power of AI in safety vigilance activities. It offers a comprehensive, scalable, and secure solution that redefines literature surveillance.