- Solutions
- AI Data and Analytics
- Demo Center
- Resources
- Company

In the intricate world of pharmacovigilance, having swift access to comprehensive statistical scores is paramount. Enter DF mSignal AI, where we seamlessly harness safety data from multiple regulatory agency portals and your company’s safety database to generate automated statistical scores.

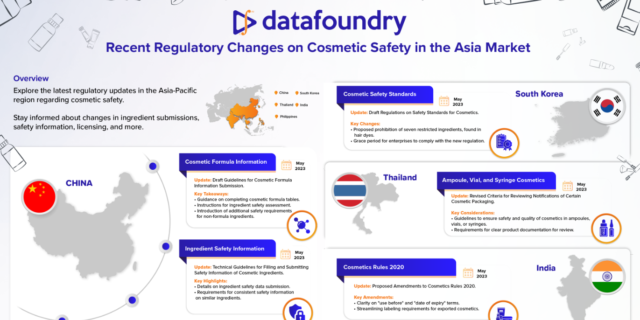
Explore the latest regulatory updates in the Asia-Pacific region regarding cosmetic safety.
Stay informed about changes in ingredient submissions, safety information, licensing, and more.

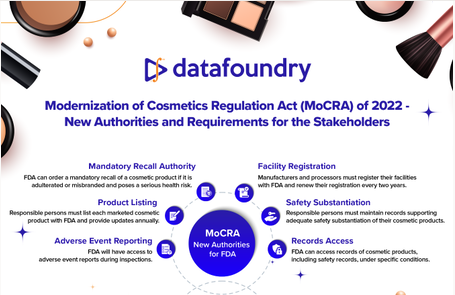
Gain valuable insights into the Modernization of Cosmetics Regulation Act (MoCRA) of 2022 through our informative infographic. This comprehensive infographic covers crucial topics such as Important Deadlines and MoCRA Timeline, Implications of MoCRA on Adverse Events Reporting, What Companies Need to Do Now, And More…..


Read our article “The Potential of Automation and AI/ML in Causality Assessment for Safety Vigilance,” featured in Pharma Focus Europe.
Explore the cutting-edge world of Automation and AI/ML, and its incredible impact on safety vigilance practices. We also delve into the challenges and opportunities that accompany the adoption of automated causality assessment, shedding light on how industry leaders can navigate this transformative landscape.


Are you a technology head, regulatory leader, or cosmetic safety professional striving to enhance the safety and compliance of cosmetic products? Look no further! Our Integrated Cosmetovigilance Solution is here to revolutionize your approach to safety case management, signal analysis, and risk assessment. Stay ahead of the curve and ensure regulatory compliance in the ever-evolving cosmetics industry.


Read about the challenges posed by data gaps in developing reliable machine learning models for pharmacovigilance, and strategies to overcome them to ultimately improve patient safety and public health in our article published in Pharmaceutical Technology Magazine, March 2023 edition.


The infographic showcases the game changing features of DF mSafety AI Ver 4.0 that helps in achieving high process efficiencies and delivers measurable value to companies in the life sciences industry.

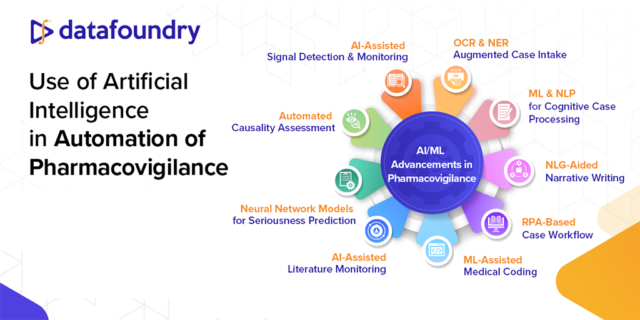
Read about how AI/ML has the potential to increase efficiency, accuracy and consistency in pharmacovigilance and can reduce costs and delivery timelines for pharmaceutical organizations in our article published in the Pharma Focus America Digital Magazine, Issue 1, 2023.


The infographic showcases the key features and benefits of DF mSignal AI and how it stands out from competitors in the market. From its ability to identify potential risks through statistical approaches, to its advanced analytics and customizable stratifications, DF mSignal AI has everything you need to stay ahead of the game.

![[04-01 15:12] Priyanka Chakraborty Priority 1: Data Digitalization Platform for Lifesciences [04-01 15:13] Priyanka Chakraborty competing against: https://www.iqvia.com/solutions/real-world-evidence/platforms/iqvia-nlp-platform](https://datafoundry.ai/wp-content/uploads/2022/09/datafoundry_new-logo_v1-1-640x320.png)
The area of Safety Vigilance (Pharmacovigilance, Cosmetovigilance and Device Vigilance) is undergoing massive transformation digitally, driven by the increasing use of Cloud Services, the rise of big data, and the adoption of artificial intelligence (AI) and machine learning (ML) technologies.


Use of AI in signal detention and Pharmacovigilance to help clinical trial manufactures, CROs, and sponsors combat drug safety-related issues and challenges

The future of life sciences is looking bright with recent developments in machine learning and artificial intelligence. AI-assisted drug discovery process has the potential to substantially reduce the time required to find new drug treatments. Using artificial intelligence, scientists can identify possible new drugs among thousands of compounds without having to do laborious, time-consuming experiments.
