
Understanding IDMP
IDMP, or Identification of Medicinal Products, is a set of five standards (ISO 11238, ISO 11239, ISO 11240, ISO 11241, ISO 11242) and four implementation guides (ISO TS 19844, ISO TS 20440, ISO TS 20443, ISO TS 20451) developed by the International Organization for Standardization (ISO).
These standards and guides provide a common data model and a standard format for the submission of data on medicinal products and substances, and they establish a unique identification for every medicinal product authorized for sale in the EU.
The main objective of IDMP is to create a unique identification for every medicinal product and to maintain a centralized database of information about all authorized medicinal products, making it easier to identify, track and recall products in case of safety concerns.
The development of IDMP standards began in the early 2000s, under the guidance of the International Organization for Standardization (ISO) and the European Medicines Agency (EMA).
The development of IDMP began in the late 2000s, with the first standard (ISO 11238:2009) being released in 2009.
Timeline of IDMP development
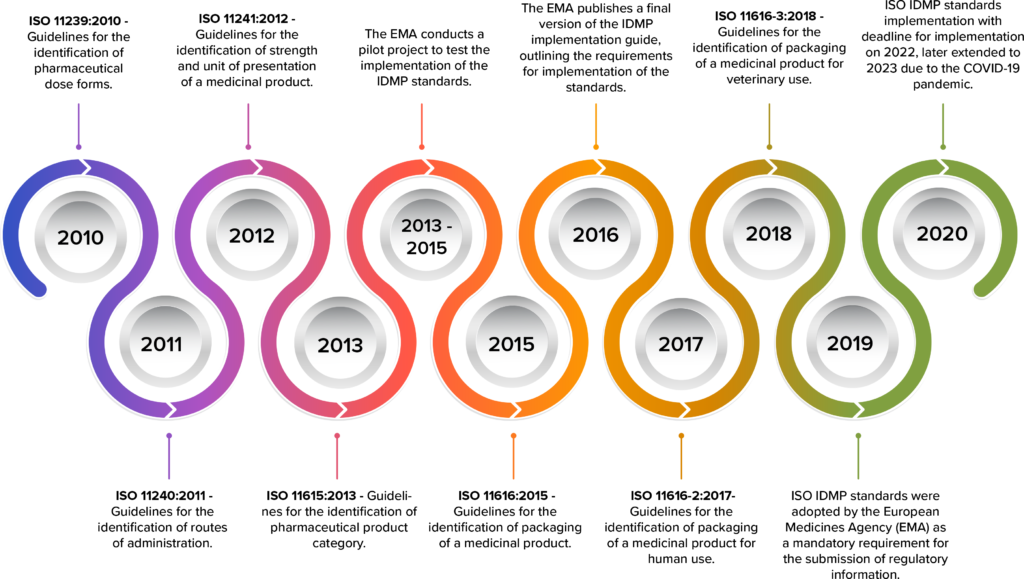
As the deadline for IDMP compliance fast approaches in 2023, pharmaceutical companies are scrambling to understand the requirements and implement the necessary changes.
IDMP compliance requires pharmaceutical companies to encode a wide range of information about their products, including the product’s name, active ingredients, and other key characteristics. This information must be organized in a way that is easy for regulatory authorities to understand and use.
IDMP compliance demands the consolidation of data from various platforms
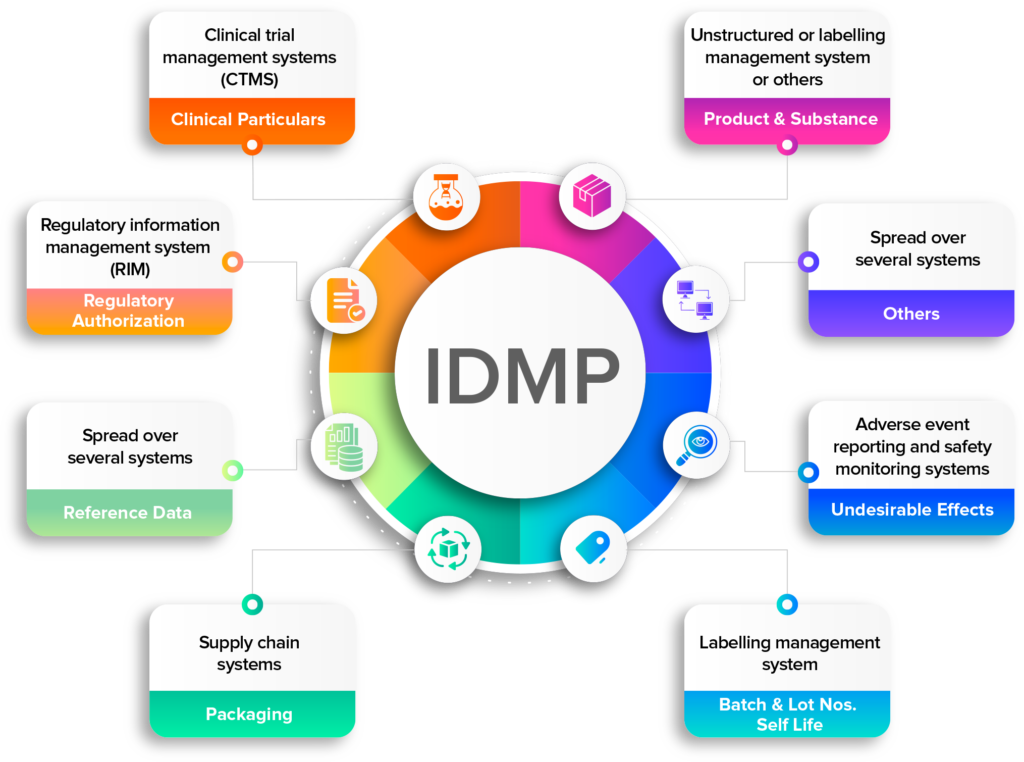
Data digitization – a prerequisite for IDMP
Data digitization is a prerequisite for IDMP (Identification of Medicinal Products) compliance because it allows companies to collect and submit the large amount of detailed information required by regulatory authorities in a digital format. This information includes data such as product names, active ingredients, dosage forms, and packaging information.
Without data digitization, it would be difficult to collect and submit this information in a format that can be easily shared and analyzed by regulatory authorities efficiently and accurately. Data digitization enables companies to quickly access, organize, and submit the required information, which can help to speed up the approval process for new drugs and medical devices.
Challenges faced for data digitization for IDMP
Pharmaceutical companies face several challenges when it comes to data digitization for IDMP compliance. One major challenge is the sheer volume of information that needs to be digitized. Many companies have large amounts of physical documents and information stored in legacy systems, unstructured regulatory documents, such as Summary of Product Characteristics (SmPCs) and eCTD Module 3 documents, which can take a significant amount of time and resources to convert into digital formats.
According to a survey conducted by IDC Health Insights –
“66% of pharmaceutical companies reported that data volume and complexity were the biggest challenges they faced in IDMP compliance.”
Companies also need to be able to demonstrate that the information they are submitting is accurate and up-to-date, which can be challenging when dealing with large amounts of data that may be stored in different systems and locations. Data digitization processes must be able to capture the required information accurately and completely, to avoid any rejection from the regulatory authorities.
Additionally, the process of data digitization for IDMP compliance requires the use of specialized software and equipment, which can be costly. Companies also need to invest in training their employees to use the software and equipment effectively, which can be time-consuming and expensive.
Moreover, the need for data privacy and security is also an important aspect that companies need to consider while digitizing their data, as they need to ensure that the sensitive information they are submitting to regulatory authorities are kept confidential.
According to another survey conducted by Deloitte-
“72% of pharmaceutical companies reported that data privacy and security were a significant concern in IDMP compliance.”
Another challenge that pharmaceutical companies face with data digitization for IDMP compliance is the need to maintain the integrity and traceability of the information. Regulatory authorities require detailed information on the origin, history, and movement of medicinal products, which can be difficult to track and maintain when dealing with large amounts of information stored in different formats.
Also, IDMP compliance requires companies to submit the data in a specific format and structure, which can be a challenge for companies that have data stored in different formats and structures. This requires companies to have a deep understanding of the IDMP standards and the ability to map their data to the required format.
Finally, IDMP standards are constantly evolving, which means that companies must be prepared to adapt to changes in order to remain compliant.
A study by Accenture found –
“Average cost of IDMP compliance for a pharmaceutical company is $8 million, with costs expected to rise in the future.”
Leveraging Technology for Effective IDMP Compliance
The implementation of IDMP can be a complex and challenging task for pharmaceutical companies, especially given the high level of data granularity required and the need to submit data to multiple systems. However, technology can play a crucial role in managing this task and making it more manageable for companies.
New technologies such as Optical Character Recognition (OCR) and Named Entity Recognition (NER) can greatly assist in fulfilling these data digitization needs for IDMP compliance.
OCR technology can automatically recognize and extract text from scanned documents, while NER can identify and extract specific information such as product names, active ingredients, and dosage forms. This can greatly reduce the need for manual data entry, reducing errors and saving time.
Additionally, AI-powered data digitization can also improve the accuracy of data extraction. Using machine learning algorithms, the system can learn to recognize specific patterns and information, which can improve its accuracy over time. The system can also be trained to recognize variations in text, such as handwriting or different font styles, which can make it more robust and less prone to errors.
In addition to automating the data extraction and coding process, technology can also help with data validation. By using machine learning algorithms, companies can quickly and accurately check the extracted and coded data for accuracy and completeness. This can help to ensure that the data is ready for submission to regulatory authorities without the need for extensive manual checking.
Overall, technology has an important role to play in helping pharmaceutical companies to comply with the IDMP requirements. By automating key tasks and providing accurate and reliable data validation, technology can help companies to save time, reduce errors, and ensure that their products are properly identified and regulated.
Key Factors to Consider for Evaluating Technology Partners for IDMP Solutions
The implementation of IDMP requires organizations to manage large amounts of data and comply with strict regulatory requirements. Therefore, it is important for organizations to choose the right technology partner to help them with the implementation of IDMP solutions.
Following are the key factors that organizations should consider when evaluating technology partners for IDMP solutions.
- Technical capabilities: The technology partner should have a strong technical team with experience in IDMP and should be able to provide solutions that are fully compliant with the IDMP standards. They should also have experience in handling complex data integration and data quality management. This includes understanding the specific data elements and data structures required for IDMP and being able to provide solutions that can handle them.
- Data management: The technology partner should have the ability to manage large amounts of data and provide solutions for data integration and data quality management. They should have experience in data governance, data modeling, data warehousing, and data migration.
- Scalability: The technology partner should be able to provide solutions that can scale to meet the organization’s needs in terms of data volume and user access. They should be able to provide solutions that can handle large data sets and support a large number of users.
- Support and maintenance: The technology partner should provide ongoing support and maintenance for their solutions. This includes providing technical support, software updates, and bug fixes. They should also have a dedicated team that can provide training and technical assistance to the organization.
- Experience: The technology partner should have relevant experience in the pharmaceutical or healthcare industry to understand the specific needs of the organization. They should have experience in implementing IDMP solutions for other companies in the industry and have a good understanding of the business processes and regulatory requirements involved.
- Security: The technology partner should have strong security measures in place to protect sensitive data and comply with data protection regulations. They should have experience in implementing security controls such as data encryption, access controls, and penetration testing.
- Cost: The technology partner should provide solutions that are cost-effective and align with the organization’s budget. They should be able to provide a detailed breakdown of the costs involved and offer flexible pricing options. They should also be transparent about any additional costs that may arise during the implementation process.
Data Digitization for IDMP Compliance – DF Digitalize AI
DF Digitalize AI helps achieve efficiency and compliance during the IDMP data life cycle. Starting from data extraction using our OCR models, to text mining using our NER models tailored for IDMP data standards, the process of generating a structured data set is automated to a large extent. The medical experts can focus on validating the output through a user-friendly UI. The IDMP data set is then made available to any downstream application such as a regulatory submission system or for analytics and insights.
Interested in data digitalization for IDMP?
Schedule a meeting with our experts.
