
Digital transformation in life sciences organizations involves implementing new technology and digital systems to improve processes and decision-making. The need for data digitalization may vary at different stages of the drug life cycle.
1. Drug discovery and pre-clinical: In the early stages of drug discovery and pre-clinical research, data digitalization can help to facilitate collaboration and information sharing among different teams and departments. Digital data can also be used to support the analysis of large and complex data sets, helping researchers to identify trends, patterns, and potential leads for new drugs.
2. Clinical development: In the clinical trials phase, data digitalization can help to ensure the accurate and timely collection, management, and analysis of data. This can be particularly important in large, multi-center clinical trials, where data from multiple sources must be integrated and analyzed. Digital data can also help to support the reporting and regulatory requirements of clinical trials, ensuring compliance and facilitating the approval process.
3. Post-approval: After a drug has been approved, data digitalization can help to support ongoing monitoring and surveillance of the drug’s safety and effectiveness. Digital data can be used to track the performance of the drug in real-world settings, and to identify any potential adverse events or trends that may require further investigation. Digital data can also help to support the ongoing reporting and regulatory requirements for approved drugs.
4. Manufacturing and commercialization: During this phase of the drug life cycle, data digitalization can help optimize the manufacturing and supply chain processes, and also the market commercialization and access of the drug product.
In summary, data digitalization can help support the efficient and effective management of data throughout the drug life cycle, from discovery and development through to post-approval monitoring and surveillance. This could enable the quality and accuracy of data, and facilitate bringing safer medicines faster to the needy patient populations
Key prerequisites for successful digital transformation
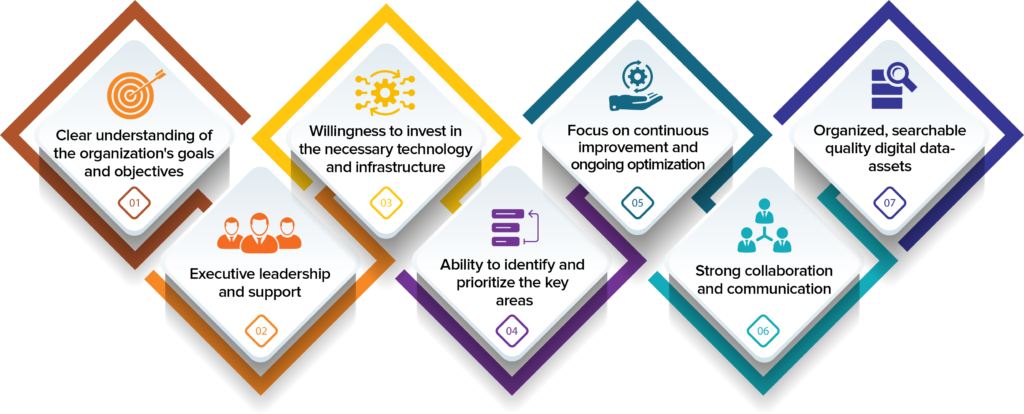
Some key prerequisites for successfully executing digital transformation include:
1. A clear understanding of the organization’s goals and objectives, and how digital technologies can help achieve them.
2. Executive leadership and support for the digital transformation initiative, with a focus on driving change and adapting to new ways of working.
3. The ability to identify and prioritize the key areas where digital technologies can have the greatest impact on the organization.
4. A willingness to invest in the necessary technology and infrastructure, including training and support for employees to help them adapt to new tools and processes.
5. A focus on continuous improvement and ongoing optimization of digital systems and processes, to ensure that they remain effective and aligned with the organization’s goals.
6. Strong collaboration and communication among teams and departments, to ensure that all stakeholders are aware of the digital transformation initiatives and can work together to support their success.
7. Finally, and most importantly- having organized, searchable quality digital data assets.
Creating organized, searchable quality digital data assets for successful digital transformation in life sciences organizations
Having organized, searchable quality digital data assets is an important prerequisite for digital transformation in life sciences organizations. This is because high-quality data is a critical input to many of the digital technologies and systems that are commonly used in the life sciences, such as artificial intelligence, machine learning, and data analytics.
Having well-organized, searchable digital data assets allows organizations to easily access the data they need to make informed decisions, improve processes, and develop new products and services. It also enables them to better manage and analyze the data, which can provide valuable insights into the organization’s operations and help identify opportunities for improvement.
To create well-organized, searchable digital data assets, life sciences organizations can use appropriate tools and systems to organize and index the digital data assets, so that they can be easily accessed and searched. In addition, organizations should consider investing in technology and expertise to help them manage and analyze their digital data assets, such as custom data management and analytics software.
Important factors to consider before investing in technology for data management and analytics software
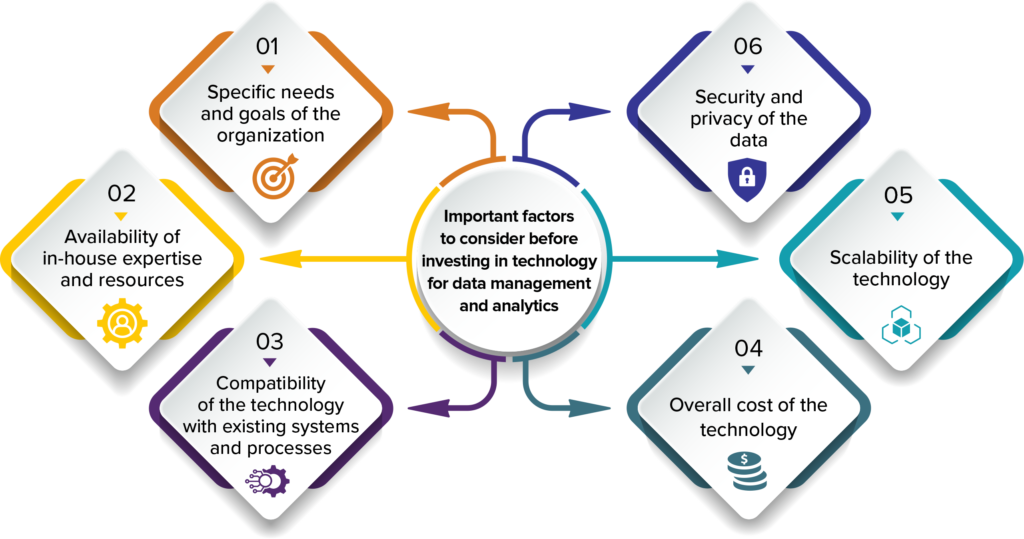
There are several important factors to consider before investing in technology for data management and analytics in the life sciences industry. Some of these factors include:
1. The specific needs and goals of the organization in terms of data management and analysis. This will help determine the type and scope of technology solutions that are most appropriate.
2. The availability of in-house expertise and resources for implementing and maintaining the technology. This may include technical staff, as well as personnel with expertise in data management and analysis in the life sciences.
3. The compatibility of the technology with existing systems and processes within the organization. It is important to ensure that any new technology can integrate seamlessly with existing systems and workflows to maximize efficiency and minimize disruptions.
4. The security and privacy of the data being managed and analyzed. Life sciences organizations often deal with sensitive and confidential data, so it is important to ensure that any technological solution can provide appropriate levels of security and privacy.
5. The scalability of the technology to accommodate future growth and changes in the organization’s data management and analysis needs. It is important to choose a solution that can adapt and evolve as the organization’s needs change over time.
6. The overall cost of the technology, including both initial investment and ongoing maintenance and support. It is important to carefully consider the potential return on investment and ensure that the technology provides a good value for the organization
Accelerating digital journey with DF Digitalize AI
At Datafoundry, our objective is to simplify and accelerate your digital transformation with the help of DF Digitalize AI solution framework. DF Digitalize AI automates the data digitization across the enterprise using best-in-class OCR, NER and Medical NLP models. The data scientists and engineers at Datafoundry will collaborate with clients’ business and IT teams to design and deploy a data fabric for your enterprise for frictionless data movement to make it easier for upstream/downstream applications to share and reuse data for process automation. Advanced AI/ML models that power DF Digitalize AI are built using a bio-medical data set of more than 10 million.
About us:
Datafoundry was founded in 2016 with the goal of simplifying data-driven business operations, by making data more accessible and easier to extract insights. We provide automation and digital transformation solutions for the life sciences and healthcare industry. Our team comprises of domain experts, data scientists and AI/ML innovators focusing on insights and automations for better health outcomes. Our flagship product DF Safety AI won India’s most prestigious AI award recently for Life Sciences. Team Datafoundry takes pride in enabling our customers to succeed – in a faster, compliant, scalable and secure manner.
