The pharmaceutical market is highly regulated, and any misstep can lead to product recall that have a multi-pronged impact. It’s important to build systems that allows one to track and monitor potential challenges.
Drug safety is a major concern in the life sciences and healthcare industry that requires constant attention and monitoring. With the rising number of complex safety cases and associated reports being submitted to regulators around the world, it has become increasingly important for drug companies to manage and submit safety data efficiently.
Use of AI/ML and automation: new age safety systems.
Life sciences and healthcare organisations are looking for new age features in their PV systems like:
• Leveraging AI/ML for Automation and Insights
• Modern, Secure & Configurable SaaS
• Reduced Costs and Improved Compliance through Automation
Industry leaders are exploring integrated approaches to Safety Case Processing and Submissions, Signal and Risk Management, Literature Monitoring and Regulatory Intelligence for Safety.
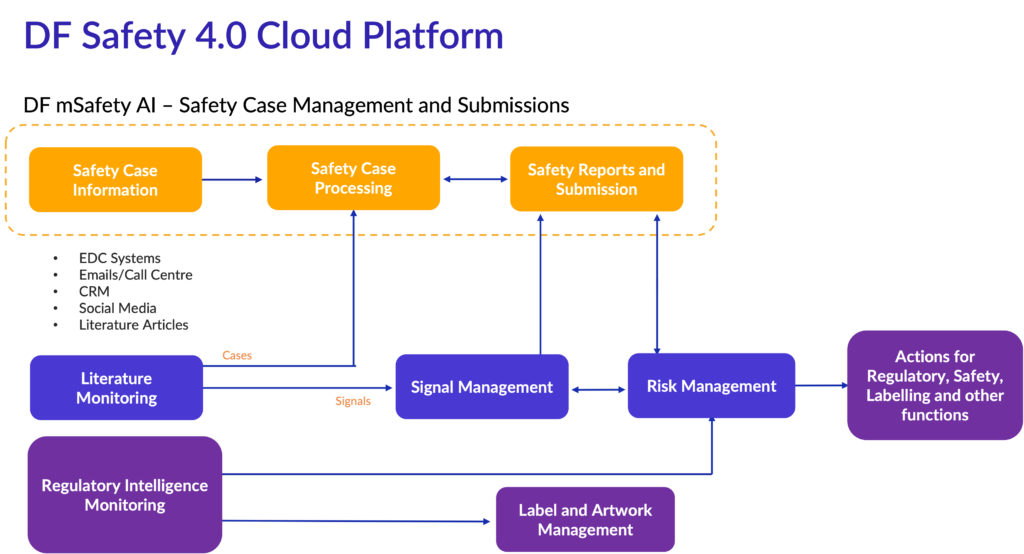
The DF Safety 4.0 is an intuitive, unified, cloud-based safety platform for safety case management through report submission.
It is a fully integrated platform of tools and functionality, offering comprehensive safety management via a clear, intuitive and user-friendly interface. DF Safety 4.0 offers the ability to import data from multiple sources and export data in various formats. It automates multiple manual processes like case intake, triage, narrative generation with integrated dictionaries, seriousness prediction and causality assessment. It has integrated PV literature monitoring and reporting functionality providing validated outputs designed for expedited and periodic reports as well as signal detection and case management. Moreover, the system offers comprehensive data visualization, including real-time alerts, dynamic drill-down reports and action plans.
Conclusion
Pharmacovigilance departments in life sciences and healthcare companies are under pressure to do more than ever with less resources. An integrated approach to managing pharmacovigilance is the key to obtaining efficiency and ensuring compliance.

